Avogadro’s law Calculator Online:
Use our Avogadro’s law Calculator Online.
Avogadro’s law Equation / formula:
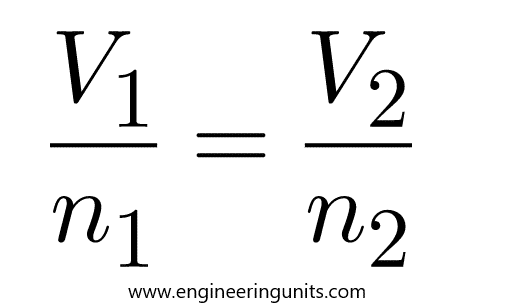
P1 / n1 = P2 /n2
P1 n2 = P2 n1
Where :
n is the amount of substance of the gas (measured in moles)
V is the volume of the gas
Avogadro’s law Equation
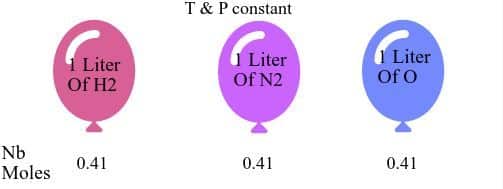
Avogadro hypothesis is that equal volume of gases contain the same number of molecules (moles) regardless of their identity. Avogadro’s Law is valid for all gases samples at constant pressure and temperature.
If you want to check the application of the avogadro’s law check the Goyal’s IIT FOUNDATION COURSE CHEMISTRY at page 168
V = constant x n
Avogadro hypothesis
Avogadro’s law Definition:
Definition of Avogadro’s law:
Avogadro’s law (sometimes referred to as Avogadro’s hypothesis or Avogadro’s principle) is an experimental gas law relating volume of a gas to the amount of substance of gas present.[1] A modern statement of Avogadro’s law is:
Avogadro’s law states that, “equal volumes of all gases, at the same temperature and pressure, have the same number of molecules”.
For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.