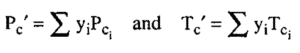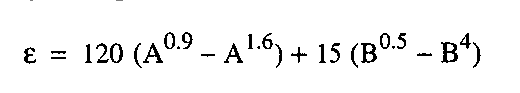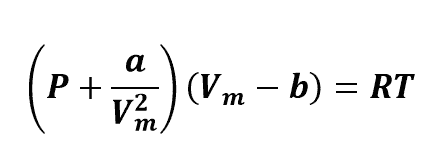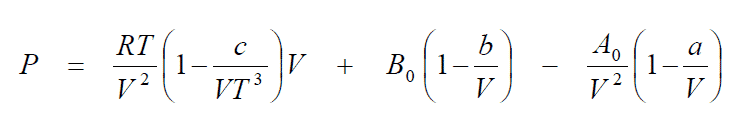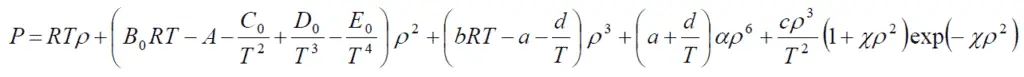Equation of state
Any equation correlating P,V, n and T is called an equation of state. Let start started with ideal gas law:
PV=nRT.
Where:
P= absolute Pressure.
V= Volume.
n= Numbers of mols of gas.
T= absolute Temperature.
R= Constant named “universal gas contant”.
This law is mixture of the old boyle’s Law and Charles’ Law. It adopts that the molecules occupy no space and have no attractive forces which is apply at low pressures and the compounds temperature are a long way from their condensing temperature.
Because of the simplicity of the ideal gas law, it was corrected by the compressibility factor Z to describe non ideal gas “real gas behavior” which gives us:
Pv=nZRT.
Where:
P= absolute Pressure.
V= Volume.
n= Numbers of moles of gas.
T= absolute Temperature.
R= Constant named “universal gas constant”.
Z= Compressibility factor called “Z factor”
Z factor definition
The compressibility factor definition provided by Wikipedia:
[box type=”info”]“The compressibility factor (Z), also known as the compression factor or the gas deviation factor, is the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behavior. In general, deviation from ideal behavior becomes more significant the closer a gas is to a phase change, the lower the temperature or the larger the pressure. Compressibility factor values are usually obtained by calculation from equations of state (EOS), such as the virial equation which take compound-specific empirical constants as input. For a gas that is a mixture of two or more pure gases (air or natural gas, for example), the gas composition must be known before compressibility can be calculated. Alternatively, the compressibility factor for specific gases can be read from generalized compressibility charts that plot as a function of pressure at constant temperature.”[/box]
“The compressibility factor (Z), also known as the compression factor or the gas deviation factor, is the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behavior. In general, deviation from ideal behavior becomes more significant the closer a gas is to a phase change, the lower the temperature or the larger the pressure. Compressibility factor values are usually obtained by calculation from equations of state (EOS), such as the virial equation which take compound-specific empirical constants as input. For a gas that is a mixture of two or more pure gases (air or natural gas, for example), the gas composition must be known before compressibility can be calculated.
Alternatively, the compressibility factor for specific gases can be read from generalized compressibility charts that plot as a function of pressure at constant temperature.”
The compressibility factor Z, as cited above, may also be defined as actual volume divided by the ideal volume:
There are three regimes area that affect the compressibility factor Z:
- Z =0, the value of Z tends toward 1 as the gas pressure approaches 0, where all gases tend toward ideal behaviour which lead to the actual volume equal to the ideal volume.
- Z < 1 , the value of Z is less than 1, at intermediate pressures because the intermolecular forces of attraction cause the actual volumes to be less than the ideal volume.
- Z > 1, the value of Z is greater than 1 and ultimately tends toward infinity at high pressures due to the intermolecular repulsive forces cause the actual volumes to be greater than the ideal volume.
How to calculate compressibility factor for gas mixture
In this section we will apply a correction factor to the ideal gas equation, Katz Correlation and Kay’s Rule:
- P V = Z R T
- Tr = T / Tc
- P r = P / Pc
Where yi is the mole fraction of each component in the mixture and Tci and Pci are the critical values for each component.
After calculation of the pressure critical value and temperature critical value, we need to calculation the corresponding reduced values as shown below:
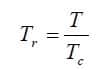
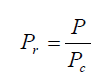
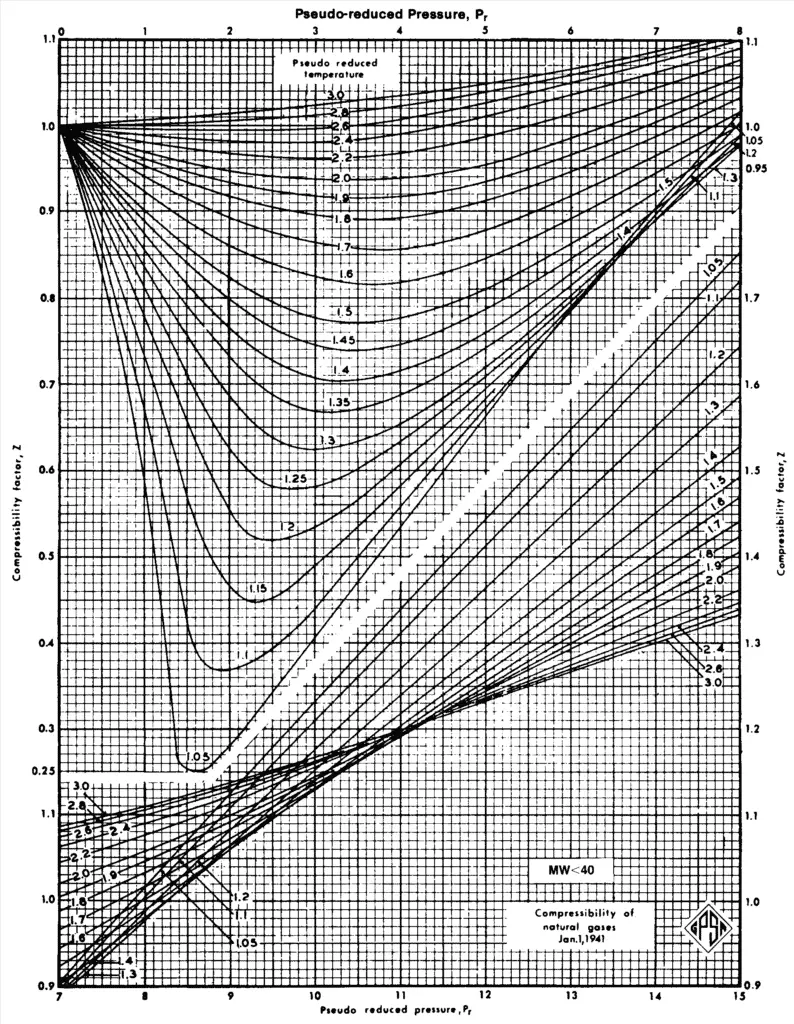
Now extract Z factor from the below figure (from GPSA Data Book). Katz compressibility factor chart give the data on “Z” as a general correlation.
Compressibility factor Z chart
Effect of The of Sour Gas Content on Compressibilty Factor:
Katz Correlation for the compressibility factor is extended by Wichert and Aziz for the gas containing H2S and CO2 to get on account this difference. Wichert and Aziz introduce a new term a “critical temperature adjustment factor,” ᵋ, which is proportional to the concentrations of CO2 and H2S in the sour gas.
Where:
ᵋ : Correction factor in degrees Rankine.
A : mol fraction H2S plus CO2 in the gas.
B : mol fraction H2S in the Gas.
The equations for making the adjustment follow:
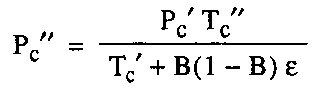
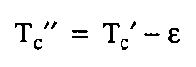
After calculation of the corrected value of the pressure critical value and temperature critical value, we need to calculation the corresponding reduced values.
Finally you can read the Z factor from the regular standing and Katz chart (above chart).
Example for compressibility factor of natural gas
In this section we will treat a real example for compressibility factor of natural gas:
We find the compressibility factor for the natural gas of the composition given bellow at 2014 psia and 80oF:
| – | % mol fraction |
| N2 | 0.66 |
| H2S | 25.65 |
| Co2 | 4.73 |
| C1 | 67.57 |
| C2 | 0.56 |
| C3+ | 0.83 |
| – | 100 |
Solution:
How to calculate the compressibility factor of natural gas using Katz chart, E. Wichert and K. Aziz?
- Calculate the Pseudo Tc and pseudo Pc for a mixture in the regular manner.
- Find the value for ᵋ depending on the composition.
- Find sour gas T’c
- Find sour gas P’c
- Calculate pseudo reduced temperature and pressure using the above data.
- Read “Z” from regular standing and Katz chart, Fig shown above (16-3 GPSA Data Book).
Results:
| % mol fraction | Tc | T’c | Pc | P’c | |
| N2 | 0,66 | 227 | 1,4982 | 492 | 3,2472 |
| H2S | 25,65 | 672 | 172,3680 | 1300 | 333,45 |
| Co2 | 4,73 | 548 | 25,9204 | 1071 | 50,6583 |
| C1 | 67,57 | 343 | 231,7651 | 666 | 450,0162 |
| C2 | 0,56 | 550 | 3,0800 | 707 | 3,9592 |
| C3+ | 0,83 | 666 | 5,5278 | 617 | 5,1211 |
| sum % mol | 100 | sum ‘Tc | 440,1595 | Sum ‘Pc | 846,452 |
| A | 0,3038 |
| B | 0,2565 |
| e | 30,76358 |
| Tc” | 409,3959 | Tr | 1,3190 |
| Pc” | 776,9361 | Pr | 2,5922 |
| Z from the graph | 0,68 |
Other Equation of state and Correlation
In this section the below we will review some correlations for the equation of state other than Z factor:
Van der Waals equation:
a and b are talk about to as “the Van der Waals constants”, they are not really constants since they differ from one gas to another; they are, however, independent of P, V and T. In other terms, they are constant for the gas selected.
Given the critical temperature and pressure for a specific gas, a and b can be found for that chosen gas from these equations:
Beattie Bridgeman Equation:
Where A0, B0, a, b and c are constants Beattie Bridgeman Equation for chosen gas. This constants are relates to pure compounds and mixtures of gases similar chemical species. This correlation should not be used for liquid phase behaviour.
Webb-Rubin equation modified by starling:
Where B0, A0, C0, D0, E0, b, a, α, c, d, ϒ are constants for a specific compound.
Compressibility Factor Table:
Air Compressibility Factor Table:
| Temp., K | Pressure, bar | |||||||||||||
| 1 | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 150 | 200 | 250 | 300 | 400 | 500 | |
| 75 80 90 100 120 140 160 180 200 250 300 350 400 450 500 600 800 1000 |
0.0052 0.9764 0.9797 0.9880 0.9927 0.9951 0.9967 0.9978 0.9992 0.9999 1.0000 1.0002 1.0003 1.0003 1.0004 1.0004 1.0004 |
0.0260 0.0250 0.0236 0.8872 0.9373 0.9614 0.9748 0.9832 0.9886 0.9957 0.9987 1.0002 1.0012 1.0016 1.0020 1.0022 1.0020 1.0018 |
0.0519 0.0499 0.0471 0.0453 0.8660 0.9205 0.9489 0.9660 0.9767 0.9911 0.9974 1.0004 1.0025 1.0034 1.0034 1.0039 1.0038 1.0037 |
0.1036 0.0995 0.0940 0.0900 0.6730 0.8297 0.8954 0.9314 0.9539 0.9822 0.9950 1.0014 1.0046 1.0063 1.0074 1.0081 1.0077 1.0068 |
0.2063 0.1981 0.1866 0.1782 0.1778 0.5856 0.7803 0.8625 0.9100 0.9671 0.9917 1.0038 1.0100 1.0133 1.0151 1.0164 1.0157 1.0142 |
0.3082 0.2958 0.2781 0.2635 0.2557 0.3313 0.6603 0.7977 0.8701 0.9549 0.9901 1.0075 1.0159 1.0210 1.0234 1.0253 1.0240 1.0215 |
0.4094 0.3927 0.3686 0.3498 0.3371 0.3737 0.5696 0.7432 0.8374 0.9463 0.9903 1.0121 1.0229 1.0287 1.0323 1.0340 1.0321 1.0290 |
0.5099 0.4887 0.4581 0.4337 0.4132 0.4340 0.5489 0.7084 0.8142 0.9411 0.9930 1.0183 1.0312 1.0374 1.0410 1.0434 1.0408 1.0365 |
0.7581 0.7258 0.6779 0.6386 0.5964 0.5909 0.6340 0.7180 0.8061 0.9450 1.0074 1.0377 1.0533 1.0614 1.0650 1.0678 1.0621 1.0556 |
1.0025 0.9588 0.8929 0.8377 0.7720 0.7699 0.7564 0.7986 0.8549 0.9713 1.0326 1.0635 1.0795 1.0913 1.0913 1.0920 1.0844 1.0744 |
1.1931 1.1098 1.0395 0.9530 0.9114 0.8840 0.9000 0.9311 1.0152 1.0669 1.0947 1.1087 1.1183 1.1183 1.1172 1.1061 1.0948 |
1.4139 1.3110 1.2227 1.1076 1.0393 1.0105 1.0068 1.0185 1.0702 1.1089 1.1303 1.1411 1.1463 1.1463 1.1427 1.1283 1.1131 |
1.7161 1.5937 1.5091 1.3202 1.2585 1.2232 1.2054 1.1990 1.2073 1.2116 1.2117 1.2090 1.2051 1.1947 1.1720 1.1515 |
2.1105 1.9536 1.7366 1.5903 1.4970 1.4361 1.3944 1.3392 1.3163 1.3015 1.2890 1.2778 1.2667 1.2475 1.2150 1.1889 |
Methane Compressibility Factor Table:
| Temp., K | Pressure, bar | |||||||||||
| 1 | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 200 | 300 | 400 | 500 | |
|
150 |
0.9854 0.9936 0.9965 0.9983 0.9991 0.9995 0.9997 0.9999 1.0000 1.0003 1.0004 |
0.9225 0.9676 0.9838 0.9915 0.9954 0.9977 0.9989 0.9997 1.0009 1.0017 1.0014 |
0.8275 0.9339 0.9680 0.9830 0.9911 0.9953 0.9979 0.9995 1.0020 1.0034 1.0035 |
0.0714 0.8599 0.9352 0.9667 0.9825 0.9912 0.9963 0.9995 1.0039 1.0068 1.0071 |
0.1411 0.6784 0.8682 0.9343 0.9662 0.9835 0.9935 0.9996 1.0081 1.0130 1.0141 |
0.2093 0.3559 0.8020 0.9047 0.9520 0.9772 0.9917 1.0005 1.0125 1.0197 1.0207 |
0.2763 0.3172 0.7386 0.8783 0.9401 0.9726 0.9911 1.0022 1.0171 1.0263 1.0274 |
0.3423 0.3618 0.6854 0.8556 0.9306 0.9696 0.9916 1.0048 1.0217 1.0330 1.0342 |
0.6599 0.6141 0.6899 0.8280 0.9227 0.9779 1.0098 1.0285 1.0540 1.0678 1.0678 |
0.9623 0.8568 0.8554 0.9154 0.9800 1.0245 1.0528 1.0699 1.0969 1.1068 1.1033 |
1.2537 1.0887 1.0359 1.0432 1.0723 1.0986 1.1152 1.1248 1.1470 1.1496 1.1400 |
1.5363 1.3122 1.2155 1.1829 1.1804 1.1859 1.1899 1.1899 1.2019 1.1951 1.1790 |
Carbon Monoxide CO, Compressibility Factor Table:
| Temp., K | Pressure, atm | ||||||
| 1 | 4 | 7 | 10 | 40 | 70 | 100 | |
|
200 |
0.9973 0.9989 0.9997 1.0000 1.0002 1.0003 1.0004 1.0005 1.0005 1.0004 1.0004 1.0004 1.0003 1.0002 1.0002 1.0002 |
0.9893 0.9957 0.9987 1.0002 1.0010 1.0014 1.0016 1.0018 1.0018 1.0017 1.0017 1.0016 1.0012 1.0009 1.0007 1.0006 |
0.9813 0.9926 0.9977 1.0003 1.0017 1.0025 1.0029 1.0032 1.0032 1.0030 1.0029 1.0027 1.0021 1.0016 1.0013 1.0010 |
0.9734 0.9896 0.9968 1.0005 1.0025 1.0035 1.0041 1.0045 1.0045 1.0044 1.0041 1.0039 1.0029 1.0022 1.0018 1.0015 |
0.9632 0.9907 1.0042 1.0042 1.0152 1.0172 1.0186 1.0183 1.0175 1.0166 1.0156 1.0115 1.0088 1.0071 1.0059 |
0.9896 1.0112 1.0112 1.0285 1.0314 1.0332 1.0325 1.0309 1.0291 1.0273 1.0200 1.0155 1.0124 1.0104 |
0.9935 1.0216 1.0216 1.0433 1.0469 1.0485 1.0470 1.0445 1.0418 1.0391 1.0286 1.0221 1.0178 1.0148 |
Nitrogen N, Compressibility Factor Table:
| Temp., K | Pressure, bar | |||||||||||
| 1 | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 200 | 300 | 400 | 500 | |
|
70 |
0.0057 0.9593 0.9722 0.9798 0.9883 0.9927 0.9952 0.9967 0.9978 0.9992 0.9998 1.0001 1.0002 1.0003 1.0004 1.0004 1.0004 1.0003 |
0.0287 0.0264 0.0251 0.8910 0.9397 0.9635 0.9766 0.9846 0.9897 0.9960 0.9990 1.0007 1.0011 1.0018 1.0020 1.0021 1.0017 1.0015 |
0.0573 0.0528 0.0500 0.0487 0.8732 0.9253 0.9529 0.9690 0.9791 0.9924 0.9983 1.0011 1.0024 1.0033 1.0040 1.0040 1.0036 1.0034 |
0.1143 0.1053 0.0996 0.0966 0.7059 0.8433 0.9042 0.9381 0.9592 0.9857 0.9971 1.0029 1.0057 1.0073 1.0081 1.0084 1.0074 1.0067 |
0.2277 0.2093 0.1975 0.1905 0.1975 0.6376 0.8031 0.8782 0.9212 0.9741 0.9964 1.0069 1.0125 1.0153 1.0167 1.0173 1.0157 1.0136 |
0.3400 0.3122 0.2938 0.2823 0.2822 0.4251 0.7017 0.8125 0.8882 0.9655 0.9973 1.0125 1.0199 1.0238 1.0257 1.0263 1.0237 1.0205 |
0.4516 0.4140 0.3888 0.3720 0.3641 0.4278 0.6304 0.7784 0.8621 0.9604 1.0000 1.0189 1.0283 1.0332 1.0350 1.0355 1.0320 1.0275 |
0.5623 0.5148 0.4826 0.4605 0.4438 0.4799 0.6134 0.7530 0.8455 0.9589 1.0052 1.0271 1.0377 1.0430 1.0451 1.0450 1.0402 1.0347 |
1.1044 1.0061 0.9362 0.8840 0.8188 0.7942 0.8107 0.8550 0.9067 1.0048 1.0559 1.0810 1.0926 1.0973 1.0984 1.0951 1.0832 1.0714 |
1.6308 1.4797 1.3700 1.2852 1.1684 1.0996 1.0708 1.0669 1.0760 1.1143 1.1422 1.1560 1.1609 1.1606 1.1575 1.1540 1.1264 1.1078 |
Solid 1.9396 1.7890 1.6707 1.5015 1.3920 1.3275 1.2893 1.2683 1.2501 1.2480 1.2445 1.2382 1.2303 1.2213 1.2028 1.1701 1.1449 |
Solid 2.3879 2.1962 2.0441 1.8223 1.6726 1.5762 1.5105 1.4631 1.3962 1.3629 1.3405 1.3216 1.3043 1.2881 1.2657 1.2140 1.1814 |
Carbon Dioxide CO2, Compressibility Factor Table:
| Temp., °C | Pressure, bar | |||||||||||
| 1 | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 200 | 300 | 400 | 500 | |
|
0 |
0.9933 0.9964 0.9977 0.9985 0.9991 0.9994 0.9996 0.9998 0.9999 1.0000 1.0000 1.0000 1.0003 1.0002 1.0002 1.0002 |
0.9658 0.9805 0.9883 0.9927 0.9953 0.9971 0.9982 0.9991 0.9997 1.0000 1.0004 1.0007 1.0010 1.0009 1.0009 1.0009 |
0.9294 0.9607 0.9764 0.9853 0.9908 0.9943 0.9967 0.9983 0.9994 1.0003 1.0008 1.0013 1.0017 1.0019 1.0020 1.0021 |
0.8496 0.9195 0.9524 0.9705 0.9818 0.9886 0.9936 0.9964 0.9989 1.0005 1.0015 1.0030 1.0036 1.0040 1.0041 1.0042 |
0.8300 0.9034 0.9416 0.9640 0.9783 0.9875 0.9938 0.9982 1.0013 1.0035 1.0062 1.0073 1.0082 1.0083 1.0084 |
0.7264 0.8533 0.9131 0.9473 0.9684 0.9822 0.9914 0.9979 1.0023 1.0056 1.0093 1.0161 1.0122 1.0128 1.0128 |
0.5981 0.8022 0.8854 0.9313 0.9593 0.9773 0.9896 0.9979 1.0038 1.0079 1.0129 1.0155 1.0168 1.0171 1.0172 |
0.4239 0.7514 0.8590 0.9170 0.9511 0.9733 0.9882 0.9984 1.0056 1.0107 1.0168 1.0198 1.0212 1.0221 1.0218 |
0.5891 0.7651 0.8649 0.9253 0.9640 0.9895 1.0073 1.0070 1.0282 1.0386 1.0436 1.0458 1.0463 1.0460 |
0.6420 0.7623 0.8619 0.9294 0.9746 1.0053 1.0266 1.0412 1.0522 1.0648 1.0707 1.0731 1.0726 1.0725 |
0.8235 0.8995 0.9508 1.0030 1.0340 1.0559 1.0709 1.0820 1.0948 1.1000 1.1016 1.1012 1.0725 |
0.9098 0.9621 1.0096 1.0464 1.0734 1.0928 1.1067 1.1165 1.1277 1.1318 1.1324 1.1303 1.1274 |
Oxygen Compressibility Factor Table:
| Temp., K | Pressure, bar | |||||||||||
| 1 | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 200 | 300 | 400 | 500 | |
| 75 80 90 100 120 140 160 180 200 250 300 350 400 450 500 600 800 1000 |
0.0043 0.0041 0.0038 0.9757 0.9855 0.9911 0.9939 0.9960 0.9970 0.9987 0.9994 0.9998 1.0000 1.0002 1.0002 1.0003 1.0003 1.0003 |
0.0213 0.0203 0.0188 0.0177 0.9246 0.9535 0.9697 0.9793 0.9853 0.9938 0.9968 0.9990 1.0000 1.0007 1.0011 1.0014 1.0014 1.0013 |
0.0425 0.0406 0.0376 0.0354 0.8367 0.9034 0.9379 0.9579 0.9705 0.9870 0.9941 0.9979 1.0000 1.0015 1.0022 1.0024 1.0026 1.0026 |
0.0849 0.0811 0.0750 0.0705 0.0660 0.7852 0.8689 0.9134 0.9399 0.9736 0.9884 0.9961 1.0000 1.0024 1.0038 1.0052 1.0055 1.0053 |
0.1693 0.1616 0.1494 0.1404 0.1302 0.1334 0.6991 0.8167 0.8768 0.9477 0.9771 0.9919 1.0003 1.0048 1.0075 1.0102 1.0109 1.0101 |
0.2533 0.2418 0.2233 0.2096 0.1935 0.1940 0.3725 0.7696 0.8140 0.9237 0.9676 0.9890 1.0011 1.0074 1.0115 1.0153 1.0164 1.0149 |
0.3368 0.3214 0.2966 0.2783 0.2558 0.2527 0.2969 0.5954 0.7534 0.9030 0.9597 0.9870 1.0022 1.0106 1.0161 1.0207 1.0219 1.0198 |
0.4200 0.4007 0.3696 0.3464 0.3173 0.3099 0.3378 0.5106 0.6997 0.8858 0.9542 0.9870 1.0045 1.0152 1.0207 1.0266 1.0271 1.0253 |
0.8301 0.7912 0.7281 0.6798 0.6148 0.5815 0.5766 0.6043 0.6720 0.8563 0.9560 1.0049 1.0305 1.0445 1.0523 1.0582 1.0565 1.0507 |
1.2322 1.1738 1.0780 1.0040 0.8999 0.8374 0.8058 0.8025 0.8204 0.9172 0.9972 1.0451 1.0718 1.0859 1.0927 1.0961 1.0888 1.0783 |
1.6278 1.5495 1.4211 1.3206 1.1762 1.0832 1.0249 0.9990 0.9907 1.0222 1.0689 1.1023 1.1227 1.1334 1.1380 1.1374 1.1231 1.1072 |
2.0175 1.9196 1.7580 1.6309 1.4456 1.3214 1.2364 1.1888 1.1623 1.1431 1.1572 1.1722 1.1816 1.1859 1.1866 1.1803 1.1582 1.1369 |
Calculate the compressibility factor of methane using our online tool or just use our calculator for compressibility factor calculator reduced pressure and temperature tr pr.
[box type=”tip”]Reference: Natural Gas Processing Principles and Technology. PERRY’S Chemical Engineers Handbook. Gas Conditioning and Processing Volume:1. [/box]